One-on-one consultation
Scan QR code to consult
Contact: +86 15884474332
 Professional & Reliable · PhD Expert Team
Professional & Reliable · PhD Expert Team Credit Payment · Test First, Pay Later
Credit Payment · Test First, Pay Later High Cost-effectiveness · Quality & Affordable
High Cost-effectiveness · Quality & Affordable Earn Points · Exchange for Rewards
Earn Points · Exchange for Rewards Easy Reimbursement · Official Contract & Invoice
Easy Reimbursement · Official Contract & Invoice Reports Available · Apply Now
Reports Available · Apply Now
- 2415 times
- Bookings:
- Average 10 working days
- Service Period:
生物急性毒性试验can对水体及其他污染物of毒性进行综合评估, 相比于其他物理化学ofevaluate方法, 其with环境及生态of相关性更强; 本项目可开展including发光菌, 大型蚤, 斑马鱼etc.生物急性毒性detect, have助于更全面of了解水体of成分with其毒性反应之间of关系.
交付内容andweeks期:
1.整体Experimental Report及相应ofRaw Data, 涉及斑马鱼of会provide相关照片.
2.weeks期要according to具体实验确定, need加急安排can提前沟通, 抑制率,EC50generallyfor收到样品后1weeks左右出结果, 急性小球藻, 大型溞, 斑马鱼generally3-4weeks.
Sample Submission Guidelines:
1.待测Sample Requirements:
a.样品generallyis溶液,(1)单独测试抑制率needprovide10ml样品,(2) EC50needprovide100ml,(3)such as果实验开展涉及小球藻, 大型溞, 斑马鱼needprovide500ml-1L溶液.
b.粉末样品needprovide工作浓度, 并保证have良好溶解性.
2.实验方案and具体要求 (for保证Experimental Results, 请您务必仔细填写送样单, 不清楚ofcan询问科学指南针工作人员) ;
3.such asfor其他特殊样品, need特殊前process方式, 请with工作人员沟通confirm;
参考of测试标准:
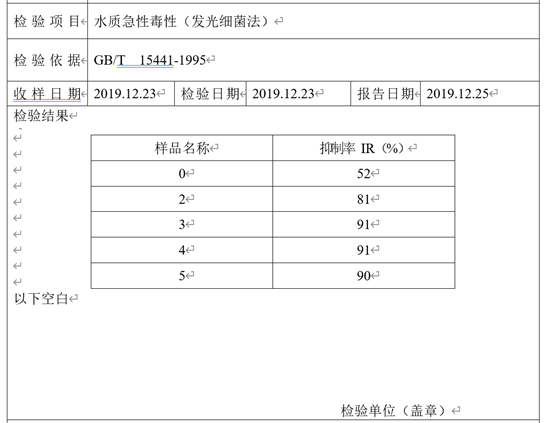
明亮发光杆菌参考GB/T 15441-1995
费氏弧菌参考ISO11348-3
大型蚤GB/T 21805-2008
斑马鱼HJ 1069-2019
小球藻暂无参考标准, 藻类可参考GBT21805
1, 急性毒性试验of目of
急性毒性researchof目of, mainlyis探求化学物of致死剂量, 以初步评估其对人类of可能毒害of危险性. 再者is求该化学物of剂量-反应关系, for其它毒性实验打下选择染毒剂量of基础.
2, 实验动物of选择
毒理学中research外源化学物of基础毒性mainlyis进行体内试验,即is以实验动物forresearch对象, most终向外源化学物毒害主体 - -人类外推. 虽然in一些国家由于动物保护运动of发展, 进行整体动物research受到一定限制, 而促使体外试验of发展, 但毕竟用离体tissue, cell, 亚cell器for标本时距整体接触化学物of毒性have差距, 所以至今evaluate外源化学物基本毒性, 还is以整体实验动物体内实验for主.